What Is The pH Level
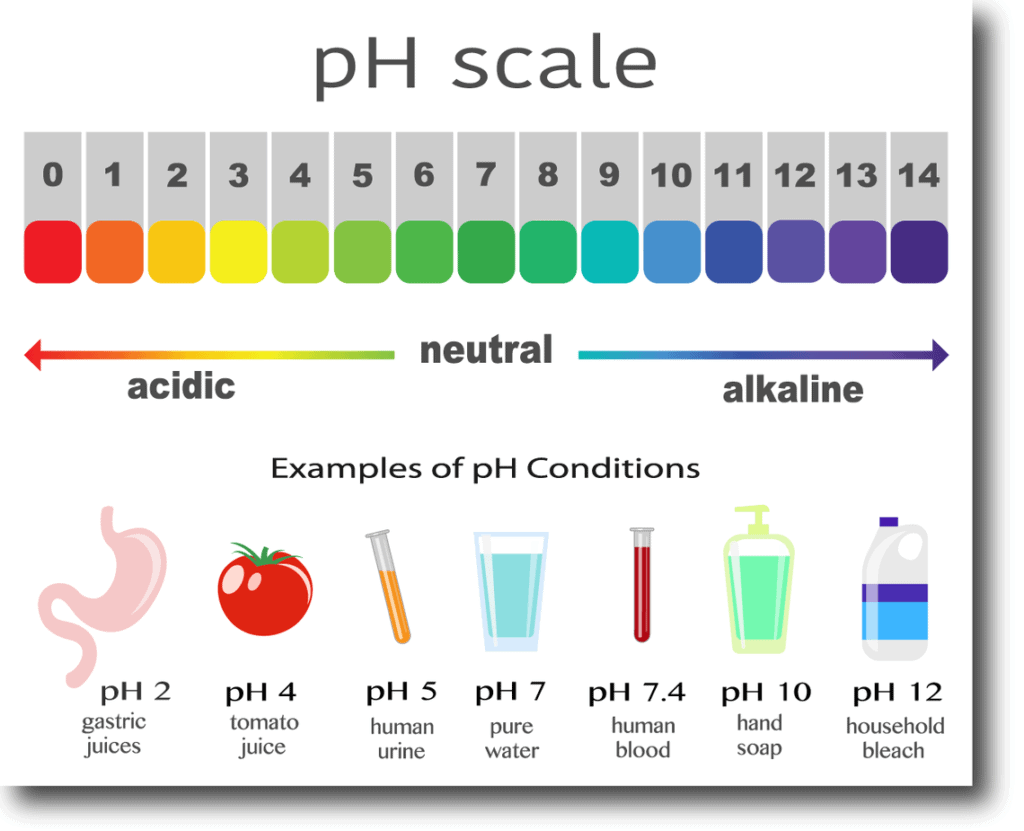
The pH level is a measure of how acidic or alkaline a substance is. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most alkaline (also known as "basic"). A pH of 7 is neutral. The pH level of a substance can affect its chemical reactivity and its biological effects.
The pH level of natural waters, such as rivers, lakes, and oceans, is typically between 6 and 8. The pH level of human blood is normally between 7.35 and 7.45. The pH level of soil can vary depending on the type of soil and the presence of organic matter.
- Discover The Unstoppable Force Of Anthony Alabi In Professional Basketball
- Unveiling The Truth Albert Pujols Baseball Saga
- Unveiling Anna Cardwells Net Worth A Journey To Financial Success
- Unveiling The Extraordinary World Of Carlos Parra A Journey Of Hyperrealism And Artistic Brilliance
- Unveiling Alvin Martin Discoveries And Insights Await
The pH level is an important factor in many chemical and biological processes. For example, the pH level of water can affect the solubility of metals and the growth of aquatic plants and animals. The pH level of soil can affect the availability of nutrients to plants and the activity of soil microorganisms.
What Is The pH Level
The pH level is a measure of how acidic or alkaline a substance is. It is an important factor in many chemical and biological processes, and it can affect the solubility of metals, the growth of aquatic plants and animals, and the availability of nutrients to plants.
- Acidity: A pH level below 7 indicates an acidic substance.
- Alkalinity: A pH level above 7 indicates an alkaline substance.
- Neutral: A pH level of 7 indicates a neutral substance.
- Solubility: The pH level of water can affect the solubility of metals.
- Aquatic life: The pH level of water can affect the growth of aquatic plants and animals.
- Nutrient availability: The pH level of soil can affect the availability of nutrients to plants.
- Chemical reactions: The pH level can affect the rate of chemical reactions.
- Biological processes: The pH level can affect biological processes, such as enzyme activity.
- Environmental health: The pH level of natural waters can be an indicator of environmental health.
The pH level is a complex topic with many implications for chemistry, biology, and environmental science. By understanding the basics of pH, we can better understand the world around us.
- Uncover The Secrets Decoding Lauren Godwins Age
- Kristen Bell Mother
- Unraveling The Mystery Uncover Amity Blights Age And Its Significance
- Discover The Extraordinary Journey Of Mitch Vogel Uncovering Hidden Insights
- Unveiling Paul Redford Beyond The Silver Screen
Acidity
Acidity is a measure of how acidic a substance is. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most alkaline (also known as "basic"). A pH of 7 is neutral. Substances with a pH below 7 are considered acidic.
Acidity is an important factor in many chemical and biological processes. For example, the acidity of water can affect the solubility of metals and the growth of aquatic plants and animals. The acidity of soil can affect the availability of nutrients to plants and the activity of soil microorganisms.
Understanding acidity is essential for understanding the pH level. The pH level is a measure of how acidic or alkaline a substance is, and it is important in many chemical and biological processes. By understanding the connection between acidity and pH, we can better understand the world around us.
Alkalinity
Alkalinity is a measure of how alkaline a substance is. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most alkaline (also known as "basic"). A pH of 7 is neutral. Substances with a pH above 7 are considered alkaline.
Alkalinity is an important factor in many chemical and biological processes. For example, the alkalinity of water can affect the solubility of metals and the growth of aquatic plants and animals. The alkalinity of soil can affect the availability of nutrients to plants and the activity of soil microorganisms.
Understanding alkalinity is essential for understanding the pH level. The pH level is a measure of how acidic or alkaline a substance is, and it is important in many chemical and biological processes. By understanding the connection between alkalinity and pH, we can better understand the world around us.
Neutral
In the context of "What Is The pH Level", understanding neutrality is crucial. A pH level of 7 signifies that a substance is neither acidic nor alkaline. This middle ground is essential for various chemical and biological processes.
- Water: Pure water has a pH of 7, indicating its neutral nature. This neutrality is vital for aquatic life, as extreme pH levels can be detrimental.
- Biological fluids: Human blood, for instance, maintains a pH close to 7. Deviations from this neutral point can indicate health issues.
- Chemical reactions: Neutral pH levels can provide optimal conditions for specific chemical reactions to occur, ensuring stability and desired outcomes.
- Environmental balance: Natural environments, such as soil and bodies of water, often strive to maintain a neutral pH level. This balance supports diverse ecosystems and ecological processes.
Comprehending the concept of neutrality is fundamental to grasp the broader concept of pH levels. It serves as a reference point against which acidity and alkalinity are measured, allowing us to understand the chemical and biological implications of varying pH levels.
Solubility
The pH level of water can significantly influence the solubility of metals. Understanding this relationship is crucial within the context of "What Is The pH Level" as it highlights the impact of pH on chemical interactions and environmental processes.
- Metal Corrosion
In acidic environments (low pH), metals tend to corrode more rapidly. This is because the hydrogen ions (H+) present in acidic solutions react with the metal surface, forming soluble metal ions. Consequently, the metal dissolves into the solution, leading to corrosion.
- Metal Precipitation
Conversely, in alkaline environments (high pH), metals may form insoluble hydroxides or carbonates. These compounds precipitate out of the solution, effectively reducing the concentration of dissolved metal ions. This process is important in water treatment plants, where pH adjustment is used to remove heavy metals from water.
- Environmental Implications
The pH level of water bodies can affect the solubility and bioavailability of metals in aquatic ecosystems. In acidic lakes and rivers, elevated levels of dissolved metals can be toxic to aquatic organisms. Conversely, in alkaline environments, metals may be less soluble and less harmful.
- Industrial Applications
The relationship between pH and metal solubility is also exploited in various industrial processes. For instance, in electroplating, the pH of the plating solution is carefully controlled to ensure the desired thickness and quality of the metal coating.
In summary, the pH level of water plays a critical role in determining the solubility of metals. Understanding this relationship is essential in fields such as chemistry, environmental science, and industrial applications. By controlling the pH, we can influence the behavior of metals in aqueous environments, mitigating corrosion, promoting precipitation, and safeguarding aquatic ecosystems.
Aquatic life
The pH level of water is a critical factor influencing the survival and well-being of aquatic organisms. As part of "What Is The pH Level", understanding this connection is vital for maintaining healthy aquatic ecosystems.
Aquatic plants and animals have specific pH ranges that support their optimal growth and reproduction. Deviations from these ranges can cause stress, reduced growth rates, impaired reproduction, and even death. For instance, acidic waters (low pH) can damage the gills of fish, making it difficult for them to breathe. Conversely, alkaline waters (high pH) can reduce the availability of essential nutrients, affecting the growth and development of aquatic plants.
The impact of pH on aquatic life highlights the importance of monitoring and regulating pH levels in water bodies. In aquaculture, maintaining optimal pH levels is crucial for maximizing fish production and ensuring their health. Similarly, in natural water systems, pH management is essential for preserving biodiversity and ecosystem balance.
By understanding the connection between pH and aquatic life, scientists, environmentalists, and water resource managers can develop stratgies to mitigate the adverse effects of pH fluctuations and protect the integrity of aquatic ecosystems.
Nutrient availability
Within the context of "What Is The pH Level", understanding the connection between soil pH and nutrient availability is essential for optimizing plant growth and agricultural productivity.
- Nutrient Uptake
Soil pH directly influences the chemical forms of nutrients in the soil, affecting their uptake by plants. For instance, in acidic soils (low pH), essential nutrients like phosphorus and iron become less soluble and less available to plants, leading to nutrient deficiencies.
- Microbial Activity
Soil pH also impacts the activity and composition of soil microorganisms, which play a crucial role in nutrient cycling. Beneficial microbes, such as nitrogen-fixing bacteria, thrive in neutral to slightly acidic soils (pH 6.0-6.8). However, extreme pH levels can inhibit their activity, reducing nutrient availability for plants.
- Soil Structure
Soil pH influences soil structure and aggregation. In acidic soils, excessive hydrogen ions can break down soil aggregates, leading to poor soil structure and reduced water and nutrient retention. This can further limit nutrient availability to plants.
- Plant Health
Optimal soil pH is essential for overall plant health and productivity. When soil pH is too low or too high, plants may exhibit symptoms of nutrient deficiencies or toxicities, affecting their growth, yield, and quality.
Understanding the relationship between soil pH and nutrient availability is crucial for developing effective soil management practices. By maintaining optimal soil pH levels, farmers and gardeners can ensure that plants have access to the nutrients they need to thrive, maximizing crop yields and promoting sustainable agricultural systems.
Chemical reactions
The pH level plays a significant role in determining the rate of chemical reactions. This relationship is crucial to understanding various chemical processes and their applications.
Chemical reactions involve the rearrangement of atoms and molecules to form new substances. The pH level, which measures the acidity or alkalinity of a solution, can influence the activity of reactants and the stability of products, thus affecting the reaction rate.
For instance, in acid-base reactions, the pH level determines the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in the solution. These ions can act as catalysts or inhibitors, speeding up or slowing down the reaction rate. Similarly, in enzyme-catalyzed reactions, the pH level can alter the ionization state of the enzyme, affecting its catalytic activity and, consequently, the reaction rate.
Understanding the connection between pH level and reaction rate is essential in various fields, including chemistry, biology, and industry. In the pharmaceutical industry, for example, controlling the pH level is crucial for optimizing drug synthesis and ensuring product quality. In environmental science, pH adjustment is used to control chemical reactions in wastewater treatment and pollution remediation.
By manipulating the pH level, scientists and engineers can fine-tune chemical processes, enhance reaction efficiency, and develop new technologies. This understanding deepens our knowledge of chemical behavior and enables us to harness it for practical applications, contributing to advancements in various scientific disciplines and industries.
Biological processes
Within the context of "What Is The pH Level", understanding the relationship between pH and biological processes is crucial, as it unravels the profound impact of pH on living organisms.
- Enzyme Activity
Enzymes are proteins that catalyze biochemical reactions in living organisms. The pH level can significantly affect enzyme activity. For instance, most enzymes have an optimal pH range within which they exhibit maximum catalytic efficiency. Deviations from this optimal range can lead to decreased enzyme activity, reduced reaction rates, and impaired cellular processes.
- Cellular Metabolism
The pH level can influence the metabolic pathways and overall functioning of cells. Extreme pH levels can disrupt cellular homeostasis, affecting membrane permeability, protein synthesis, and energy production. Maintaining optimal pH levels is essential for proper cellular function and survival.
- Signal Transduction
pH changes can serve as signaling molecules, triggering specific responses within cells and tissues. For example, in the nervous system, pH changes can modulate neurotransmitter release and neuronal excitability, influencing signal transduction and information processing.
- Immune Function
The pH level plays a role in the regulation of immune responses. For instance, in the phagocytic process, acidic pH within phagolysosomes is crucial for the killing of ingested pathogens. Alterations in pH levels can impair immune cell function and contribute to disease susceptibility.
Comprehending the connection between pH and biological processes enhances our understanding of the intricate workings of living systems. By manipulating pH levels, scientists and researchers can gain insights into disease mechanisms, develop new therapeutic strategies, and explore the potential of pH-responsive materials in biomedical applications.
Environmental health
The pH level of natural waters, such as rivers, lakes, and oceans, is a crucial indicator of environmental health. It provides valuable insights into the overall well-being of aquatic ecosystems and the potential risks to human health.
When the pH level of natural waters deviates significantly from the optimal range, it can disrupt the delicate balance of aquatic ecosystems. Acidic waters (low pH) can harm aquatic organisms, damage their habitats, and release harmful metals into the environment. Conversely, alkaline waters (high pH) can promote the growth of nuisance algae and reduce the solubility of essential nutrients.
Monitoring the pH level of natural waters is essential for assessing the health of aquatic ecosystems and implementing appropriate management strategies. It helps identify sources of pollution, such as acid rain or industrial discharges, and enables the development of mitigation measures to restore and protect water quality.
By understanding the connection between the pH level of natural waters and environmental health, we can make informed decisions to protect our aquatic resources and ensure the well-being of both human and aquatic life.
FAQs about pH Levels
The pH level is a crucial indicator of the acidity or alkalinity of a substance. Here are answers to some frequently asked questions about pH levels:
Question 1: What is the pH scale?
The pH scale is a logarithmic scale used to measure the acidity or alkalinity of a substance. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline (also known as "basic"). A pH of 7 is neutral.
Question 2: What is a neutral pH?
A neutral pH is a pH of 7. This means that the substance is neither acidic nor alkaline. Pure water has a neutral pH.
Question 3: What is an acidic pH?
An acidic pH is a pH below 7. This means that the substance has a higher concentration of hydrogen ions (H+) than hydroxide ions (OH-).
Question 4: What is an alkaline pH?
An alkaline pH is a pH above 7. This means that the substance has a higher concentration of hydroxide ions (OH-) than hydrogen ions (H+).
Question 5: How can I measure the pH of a substance?
There are several ways to measure the pH of a substance. One common method is to use a pH meter, which is an electronic device that measures the electrical potential of a solution and converts it to a pH reading.
Question 6: Why is pH important?
pH is important because it affects many chemical and biological processes. For example, the pH of water can affect the solubility of metals, the growth of aquatic plants and animals, and the activity of soil microorganisms.
Understanding pH levels is essential for a variety of fields, including chemistry, biology, environmental science, and agriculture.
Summary:
- The pH scale measures the acidity or alkalinity of a substance.
- A neutral pH is 7, while an acidic pH is below 7 and an alkaline pH is above 7.
- pH can be measured using a pH meter.
- pH is important because it affects many chemical and biological processes.
Transition to the next article section:
Now that we have a better understanding of pH levels, let's explore some specific examples of how pH affects our world.
Tips to Understand pH Levels
Understanding pH levels is crucial in various fields, including chemistry, biology, and environmental science. Here are a few tips to help you grasp this important concept:
Tip 1: Remember the pH Scale
The pH scale ranges from 0 to 14, with 7 being neutral. Values below 7 indicate acidity, while values above 7 indicate alkalinity.
Tip 2: Understand the Impact on Chemical Reactions
pH levels can significantly influence the rate and outcome of chemical reactions. For instance, acidic conditions promote reactions involving hydrogen ions, while alkaline conditions favor reactions involving hydroxide ions.
Tip 3: Consider Biological Implications
pH levels play a vital role in biological processes. For example, the pH of blood is tightly regulated to maintain optimal enzyme activity and cellular function.
Tip 4: Measure pH Accurately
Accurate pH measurement is essential. Use calibrated pH meters or reliable test kits to obtain precise readings.
Tip 5: Explore Real-World Applications
Understanding pH levels has practical applications in industries such as agriculture, water treatment, and medicine. By manipulating pH, scientists and engineers can optimize processes and improve product quality.
Summary:
- Familiarize yourself with the pH scale and its implications.
- Recognize the influence of pH on chemical reactions and biological processes.
- Utilize accurate methods to measure pH levels.
- Explore real-world applications of pH manipulation.
By following these tips, you can enhance your understanding of pH levels and their significance in various scientific disciplines and practical applications.
Conclusion
In exploring "What Is The pH Level", we have gained insights into its profound significance in chemistry, biology, and environmental science. pH levels provide crucial information about the acidity or alkalinity of substances, offering valuable perspectives on their behavior and interactions.
The pH scale serves as a universal measure, enabling scientists and researchers to quantify and compare the acidity or alkalinity of diverse substances. Understanding pH levels is essential for unraveling the mechanisms of chemical reactions, assessing biological processes, and evaluating environmental health. By manipulating pH, we can optimize industrial processes, develop innovative technologies, and address environmental challenges.
As we continue to explore the intricacies of pH levels, new discoveries and applications will undoubtedly emerge. Embracing a deeper understanding of this fundamental concept empowers us to make informed decisions, foster scientific advancements, and contribute to a more sustainable and prosperous future.
Related Resources:
- Unveiling Caribe Devines Net Worth Secrets And Surprises Revealed
- Unveiling Central Cees Net Worth Secrets And Insights For 2024
- Discover The Secrets Of January Jones Modeling Success
- Unveiling The Secrets Of Sami Maleks Height Insights And Revelations
- Unveiling Leslie Whitaker The Accomplished Journalist And Wife Of Bill Whitaker

Detail Author:
- Name : Ms. Margarete Howell III
- Username : olaf86
- Email : delbert05@welch.com
- Birthdate : 1984-03-23
- Address : 5249 Stracke Locks East Loyce, KY 71126
- Phone : 1-352-664-2737
- Company : Nolan Ltd
- Job : Continuous Mining Machine Operator
- Bio : Ipsa est aut maxime consequatur dolor vero dicta. Animi sed unde sapiente excepturi id quo. Voluptates et inventore quia enim cum. Consequuntur tempora nesciunt quo repudiandae.
Socials
twitter:
- url : https://twitter.com/rosamond_real
- username : rosamond_real
- bio : Et sunt quo et assumenda quidem aliquam porro. Cupiditate quo omnis nihil quis vitae et mollitia. Fugit eveniet enim ex quo magni magnam fugit.
- followers : 5298
- following : 2924
linkedin:
- url : https://linkedin.com/in/rhoeger
- username : rhoeger
- bio : Autem voluptatem doloremque ut commodi provident.
- followers : 6434
- following : 259
instagram:
- url : https://instagram.com/hoegerr
- username : hoegerr
- bio : Et illum dolor illo sunt. Placeat fuga maiores molestiae tenetur nihil in aut. Et est ab autem.
- followers : 6112
- following : 1025